The department of agriculture has dismissed what it calls “misleading and hyperbolic” claims that South Africa’s agricultural inputs regulatory system is on the brink of collapse. This follows a wave of public statements and a legal letter from the lobby group SAAI alleging a “persistent collapse” in the administration of Act 36.
This is the law governing the registration of pesticides, fertilisers, farm feeds and stock remedies.
Food For Mzansi has seen the department’s detailed response, which paints a very different picture: one of a regulatory system under pressure, yes, but also one in the midst of a significant modernisation drive.
Digital overhaul under way
Upon taking office, agriculture minister John Steenhuisen elevated the Act 36 backlog as a priority issue. Since then, the department says it has made “significant inroads” in modernising the system, most notably with the launch of a new online application portal for pesticides in December 2023.
This digital platform allows applicants to submit documents electronically, track the status of their applications in real time, and generate reports, including lists of registered pesticides. For an industry long accustomed to manual submissions and long queues at departmental offices, the shift is substantial.
“By going digital, the department is eliminating unnecessary delays and creating a ‘fast track’ for companies that comply with requirements from the start,” Steenhuisen said, noting that this aligns with his earlier commitments made on 2 October 2025.
From 1 April 2026, the department will no longer accept manual pesticide applications. The digital rollout will then expand to fertilisers, stock remedies and animal feeds.
📢 Stand Up, Be Seen, Be Counted
We want to provide you with the most valuable, relevant information possible. Please take a few minutes to complete this short, confidential survey about your farming practices and challenges. Your feedback helps us tailor our coverage to better support the future of agriculture across Mzansi.
Processing thousands of applications
Contrary to allegations of administrative breakdown, the Office of the Registrar has processed and finalised 6 617 applications in the 2024/25 financial year alone. Over the last five years, 51 165 of 56 890 applications received have been completed.
Current turnaround times range from two weeks to 24 months, depending on application complexity.
To manage demand, the department has boosted internal capacity with consultants and is filling two key scientific posts left vacant due to resignations. These positions are crucial to the technical evaluation process.
Backlog remains, but ‘collapse’ it is not
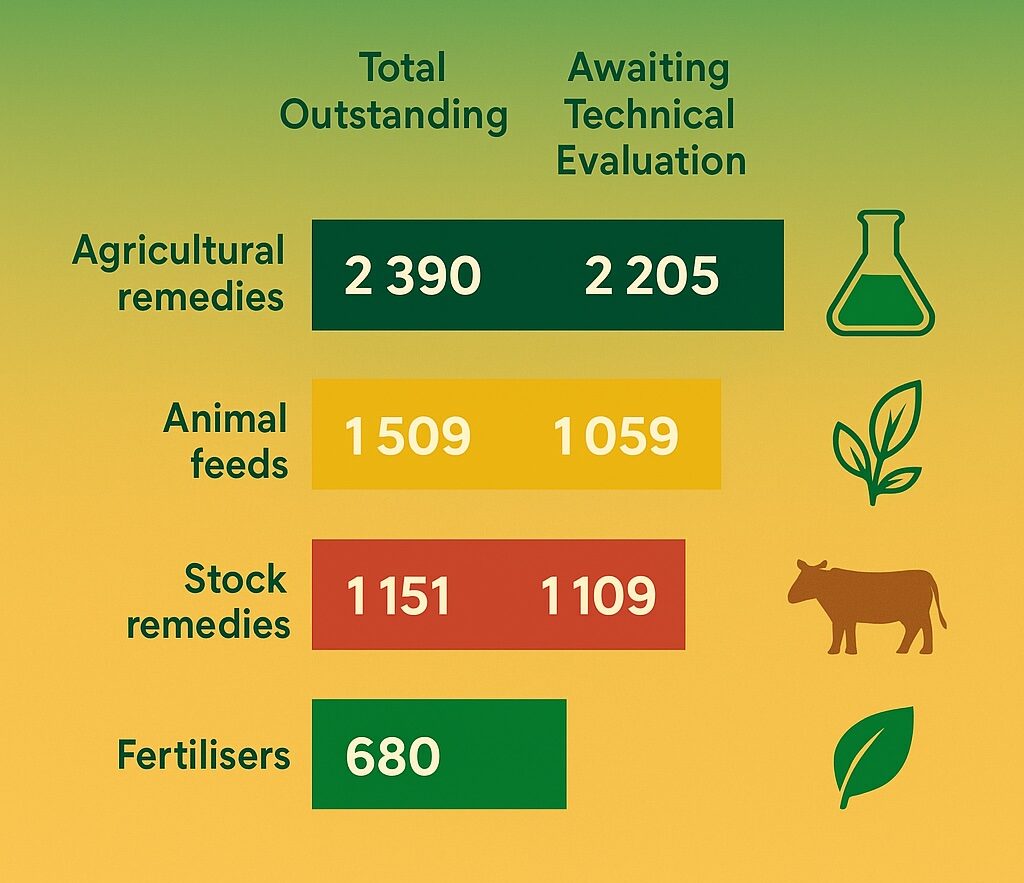
The total outstanding backlog sits at 5 730 applications, most of which (about 4 800) are awaiting technical evaluation. The department attributes this mainly to a surge in applications, incomplete submissions from industry, concurrence requirements from other government entities, and slow applicant responses.
A category breakdown shows:
- Agricultural remedies: 2 390 outstanding (2 205 needing technical evaluation)
- Animal feeds: 1 509 outstanding (1 059 awaiting evaluation or approval)
- Stock remedies: 1 151 outstanding (1 109 awaiting evaluation)
- Fertilisers: 680 outstanding (442 awaiting technical evaluation)
A “time-bound action plan” to clear the backlog and stabilise the registrar’s office is under development.
Rift Valley Fever vaccines: No shortage, says OBP
With livestock farmers anxious amid reports of Rift Valley Fever (RVF) outbreaks, Onderstepoort Biological Products (OBP) has moved to correct rumours of vaccine shortages. According to OBP, there are currently 2.4 million live RVF vaccine doses in stock, in addition to the following quantities already distributed since the start of the outbreak:
- RVF (live): 465 200 doses
- RVF (inactive): 118 050 doses
Production for December 2025 is projected at 2.6 million live doses and 375 000 inactive doses, with an additional 1.5 million Clone 13 doses to supplement the inactive segment. OBP plans to manufacture 9 million RVF doses between January and March 2026.
The department confirms that all critical remedies required for biosecurity and food security have been registered. One pending RVF vaccine application is currently tied up in a court process, while the other is being reviewed and is expected to be finalised within about three weeks.
Industry urged to work with facts, not fear
While acknowledging that frustrations exist, the department insists that claims of a regulatory “collapse” are deeply exaggerated.
The real challenge, it says, lies not in administrative dysfunction but in a capacity crunch driven by scientific evaluation demands; an issue it is actively addressing.
As Steenhuisen put it: the path forward is about “efficiency, accountability and transparency”, with digital transformation at the centre.
READ NEXT: Engineering graduate takes shot left to farming in North West

















